
Has an active autoimmune disease that has required systemic treatment in the past 2 Has not recovered (e.g., to ≤ Grade 1 or to baseline) from AEs due to a previouslyĪdministered therapy. Received an investigational agent and/or used an investigational device within 4 weeks Exclusion Criteria: - Is currently participating in a clinical study and receiving an investigational agentĪnd/or using an investigational device, or has participated in a clinical study and
Keynote 355 pembrolizumab plus#
Sperm during the intervention period and for at least the time needed to eliminateĮach study intervention after the last dose of study intervention PLUS be abstinentįrom heterosexual intercourse OR must agree to use contraception unless confirmed toīe azoospermic. Male participants are eligible to participate if they agree to refrain from donating Local regulations) within 24 hours (urine) or 72 hours (serum) before the first dose Needed to eliminate each study intervention after the last dose of study interventionĪND has a negative highly-sensitive pregnancy test ( as required by Heterosexual intercourse during the intervention period and for at least the time Using a contraceptive method that is highly effective or is abstinent from Female participants are eligible to participate if they are not pregnant orīreastfeeding AND they are not a woman of childbearing potential (WOCBP) OR is a WOCBP Demonstrates adequate organ function, within 10 days prior to the start of study drug. Has a life expectancy ≥12 weeks from randomization. Has an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, asĪssessed within 10 days prior to the start of study drug. Status and PD-L1 expression, unless contraindicated due to site inaccessibility and/or Recurrent inoperable or metastatic tumor lesion for central determination of TNBC Has provided recently or newly obtained core or excisional biopsy from a locally Has measurable disease based on Response Evaluation Criteria in Solid Tumors versionġ.1 (RECIST 1.1) as determined by local radiology review. In the (neo)adjuvant setting, unless anthracycline was contraindicated or notĬonsidered the best treatment option for the participant in the opinion of the Has been treated with (neo)adjuvant anthracycline, if they received systemic treatment Whichever occurred last) and first documented local or distant disease recurrence. Primary breast tumor surgery or date of last adjuvant chemotherapy administration, Has completed treatment for Stage I-III breast cancer, if indicated, and ≥6 monthsĮlapsed between the completion of treatment with curative intent (e.g., date of Has centrally confirmed TNBC, as defined by the most recent American Society ofĬlinical Oncology/college of American Pathologists (ASCO/CAP) guidelines. Inclusion Criteria: - Has locally recurrent inoperable breast cancer not previously treated withĬhemotherapy and which cannot be treated with curative intent OR has metastatic breastĬancer not previously treated with chemotherapy. participants with PD-L1 CPS ≥10 tumors. participants with PD-L1 CPS ≥1 tumors, and the combination of pembrolizumab and chemotherapy prolongs Overall Survival (OS) participants with PD-L1 CPS ≥10 tumors, andĢ.
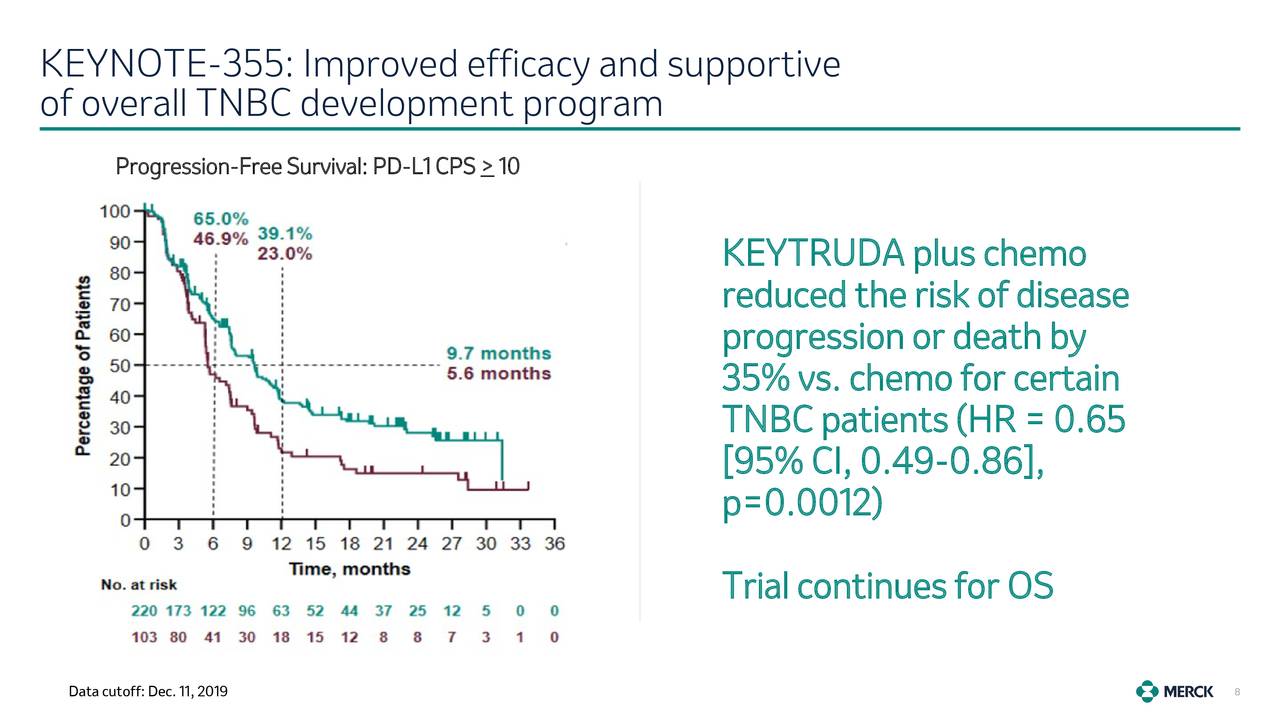
participants with programmed cell death-ligand 1 (PD-L1) combined positive score (PFS) compared to placebo and chemotherapy in: the combination of pembrolizumab and chemotherapy prolongs Progression-Free Survival Treatment of locally recurrent inoperable or metastatic TNBC, which has not been previouslyġ. In Part 2, the safety and efficacy of pembrolizumab plus background chemotherapy will beĪssessed compared to the safety and efficacy of placebo plus background chemotherapy in the Metastatic triple negative breast cancer (TNBC), which has not been previously treated with In Part 1, the safety of pembrolizumab (MK-3475) in combination with one of three differentĬhemotherapies will be assessed in the treatment of locally recurrent inoperable or A Randomized, Double-Blind, Phase III Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple Negative Breast Cancer - (KEYNOTE-355)


 0 kommentar(er)
0 kommentar(er)
