
The reactivity of a metal is related to its tendency to form positive ions. At the top, we have the most reactive metals and the least reactive.
Reactivity series chemistry gcse series#
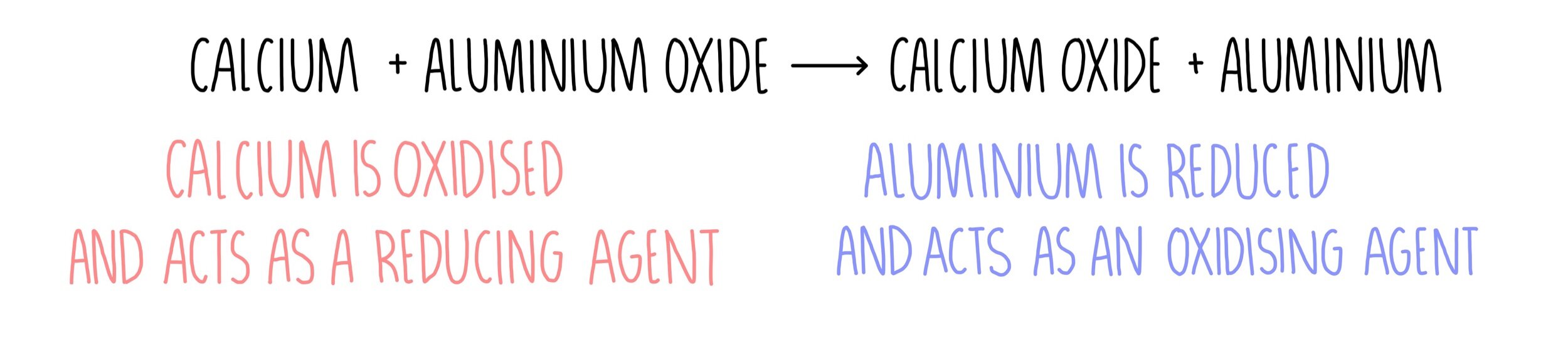
Understanding the reactivity series is fundamental to chemistry it explains why most reactions happen and what changes the particles will undergo during the reaction. GCSE AQA Trilogy Reactions of metals - AQA The reactivity series shows metals in order of reactivity. The reactivity series is a list of metals which are arranged in order of their reactivity. Use the results in the table to deduce an order of reactivity, starting with the most reactive metal. The reactivity series lists elements (mostly metals) in order of decreasing reactivity. The table shows the results of one of these investigations. Different combinations of metal and salt solution are tested. A piece of metal is dipped into a salt solution. the blue colour of the solution fades as blue copper sulfate solution is replaced by colourless magnesium sulfate solutionĪ reactivity series can be deduced by carrying out several displacement reactions.Mg(s) + CuSO 4 (aq) → MgSO 4 (aq) + Cu(s) Magnesium + copper sulfate → magnesium sulfate + copper It displaces copper from copper sulfate solution : For example, magnesium is more reactive than copper. Displacement in solutionsĪ more reactive metal can displace a less reactive metal from its compounds. At the same time, copper oxide is reduced because oxygen is removed from it. The reactivity series shows the list of metals starting with the most reactive at the top and the least reactive at the bottom. In this reaction, carbon is oxidised because it gains oxygen. For example:Ĭopper oxide + carbon → copper + carbon dioxide It is a watered-down version of the Electrochemical Series. Oxygen can be removed from metal oxides in chemical reactions. The reactivity series of metals shows how reactive some elements are compared with others. The reactions are oxidation reactions because the metal gains oxygen. For example, magnesium burns rapidly in air: Many metals react with oxygen to make metal oxides. Suggest why.Oxidation, reduction and displacement reactions Reactions of metals with oxygen Some students did not test combinations of the same metal, such as magnesium and magnesium sulfate solution. If you are struggling to remember the reactivity series, this song (warning its quite catchy) might help you Reactivity Series song. write equations for any reactions that occurred Introduction into using the reactivity series and making predictions for chemical reactions.use the results to construct a reactivity series for the metals used.describe any changes in the colour of the metal or the metal salt solution.

The rhyme will help you to remember the order, but in most. The metals in this list are ordered from most reactive to least reactive There are 3 main things to know about the picture of the reactivity series above. The reactivity series is just a list of metals. Reacting metals with metal salt solutions Results Why is it useful to label where carbon and hydrogen come on the reactivity series.
This is an outline of the required steps to undertake one of these methods. There are a number of ways that you could investigate a displacement reaction. Metal displacement reactions practical Displacement in solutions


 0 kommentar(er)
0 kommentar(er)
